-
Notifications
You must be signed in to change notification settings - Fork 38
ExplicitHs
Greg Landrum edited this page Dec 4, 2019
·
2 revisions
When Hs are removed from a molecule, all Hs are removed except:
- Hs where an isotope has been set
- Hs that have a wedged or dashed bond to them
- Hs bonded to atoms with tetrahedral stereochemistry set ("Chiral Hs")
- Hs bonded to atoms with three (or more) ring bonds that are not simply protonated
- Hs bonded to atoms in a non-default valence state that are not simply protonated
For the above, the definition of "simply protonated" is an atom with charge = +1 and a valence that is one higher than the default.
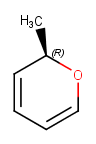
Here are some examples
| Original | Standardized | Notes |
|---|---|---|
 |
 |
|
 |
 |
|
 |
 |
|
 |
 |
Only noncyclic bond is to a chiral center |